Advarra In Conversations With ...
Episodes of Advarra In Conversations With ...
Mark All
Through their real-world experiences and shared insights, Jonathan Aguilar from The University of Texas MD Anderson Cancer Center and Advarra's Erick Sperloen illustrate how a blended approach transcends the limitations of standalone eLearning
DCTs are more than just technology! In this episode, we discuss the evolution of decentralized trial modalities, tailoring clinical trials for the participant population, and possible solutions to regulatory challenges.
How does single IRB (sIRB) actually work in practice? In this episode, you’ll get the inside scoop on how institutions implement sIRB requirements and overcome the potential challenges of relying on an outside IRB.
What is trending in the clinical research industry? In this podcast, we discuss our predictions and perspectives on the 2023 clinical research trends.
In this episode, we discuss the current state of diversity, equity, and inclusion efforts in research, how technology can drive inclusivity in clinical trials, and the role of sites in engaging diverse participants.
In this episode of Advarra’s new series “My Favorite Case Study,” we discuss patient-centric strategies and solutions of real research programs at CSL Behring to improve the patient experience and retention in clinical trials.
In the kick-off episode of Advarra’s new series “My Favorite Case Study,” we explore unique ethical considerations for current monkeypox research efforts and how the COVID-19 pandemic has shaped vaccine development, promoting accelerated resear
In this episode, we discuss workforce challenges in the clinical research industry and the impact of the great resignation.
In this episode, we explore the ethics and implications of artificial intelligence in clinical research.
In this podcast episode, Advarra’s Robann Cunningham and the Patient Empowerment Network’s Robin Barnes discusses patient empowerment and advocacy.
In this episode, Advarra's IRB Chairs Luke Gelinas and Amanda Higley discuss hot topics in clinical research, including cannabis studies, returning research results, and data ownership.
What will be the 2022 trends in clinical research? In this episode, Advarra's James Riddle and Wendy Tate forecast what is coming up for clinical trials and clinical research in 2022, including changes in study design, gene therapy innovations,
In this episode, Advarra's James Riddle and Willie Muehlhausen, Co-Founder of SAFIRA Clinical Research, discuss the growth of decentralized clinical trial technology and how IRBs evolved to account for technology advancements.
In this episode, Wendy Tate chats with Tiffany Danielle Pineda and Brian Sevier to discuss what the University of Florida Clinical and Translational Science Institute has accomplished to increase diversity, equity, and access in clinical resear
The Common Rule provides a framework for ethical conduct of clinical research involving human participants. In this episode, Advarra’s Lauren Hartsmith and Connie Cullity dive into what the Common Rule is and what it means for sponsors, investi
Discussions with:Wendy TatePhD, MS, GStatSenior Clinical Research StrategistAdvarraDylan RosserExecutive Director, Global Development OperationsAmgen
Discussions with:James RiddleMCSE, CIP, CPIA, CRQMVP of Research Services & Strategic ConsultingAdvaraBarbara SchneiderPh.D., MBAExecutive Director of Biostatistical ServicesAdvarra
Patient advocates and industry innovators Aidan Gannon of Advarra and Jessica Perry of Moderna discuss patient centricity in clinical research, examine COVID-19's impact on patient-centric conduct, and investigate evolving expectations of trial
In this episode, Jake Meyer and Candi Barlow review results from Advarra's recent survey and discuss study activation success strategies for sites. Our experts share their thoughts on budget negotiation from how to make do with a tight budget t
The Gene Therapy market is accelerating at unprecedented speed, with expected growth globally of 16.6% between 2020-2027, but it hasn’t been an easy road. In this episode, Advarra’s Daniel Eisenman talks with Dr. Adam Soloff and Dr. Paul Gulig
Diversity, equity, and inclusion (DEI) in cancer research are critical to clinical research. In this episode, Advarra’s Cheryl Byers and Dr. John H. Stewart have a discussion on what is DEI, the current climate of DEI in cancer research, and co
In this episode, Advarra’s Vice President of Research Services & Strategic Consulting, James Riddle, is joined by Advarra IRB Chair, Luke Gelinas, in an enriching discussion on digital health and regulation in clinical trials. Riddle and Gelina
Unlock more with Podchaser Pro
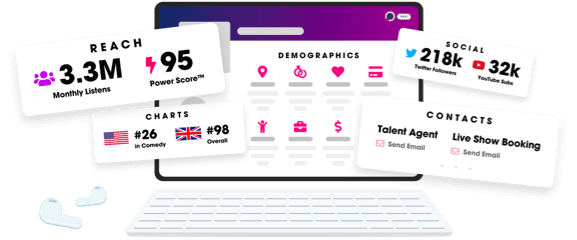
- Audience Insights
- Contact Information
- Demographics
- Charts
- Sponsor History
- and More!
- Account
- Register
- Log In
- Find Friends
- Resources
- Help Center
- Blog
- API
Podchaser is the ultimate destination for podcast data, search, and discovery. Learn More
- © 2025 Podchaser, Inc.
- Privacy Policy
- Terms of Service
- Contact Us
